Bethany Pan,, PharmD, Ryan Beechinor, PharmD, BCPS, BCOP
Metastatic melanoma presents as a highly immunogenic form of cancer characterized by extensive mutational load and variations within and between tumors. This unique immunogenic nature has facilitated its response to therapies utilizing T-cells such as immune checkpoint inhibitors. While checkpoint inhibitors (programmed cell death protein 1 [PD-1], cytotoxic T-lymphocyte-associated antigen 4 [CTLA-4], and lymphocyte activation gene-3 [LAG-3] inhibitors) and targeted therapy (rapidly accelerated fibrosarcoma B-type [BRAF]/mitogen-activated extracellular signal-regulated kinase [MEK] inhibitors) have transformed the management of advanced melanoma, recurrence remains frequent, and there are limited treatment options for patients who have failed first-line therapies.1
Lifileucel (Amtagvi, Iovance Biotherapeutics), an autologous tumor-infiltrating lymphocyte (TIL) therapy, was the first of its kind to be approved to treat cancer and the first cellular therapy to be approved for a solid tumor.2 The FDA accelerated approval granted use of lifileucel for adults with metastatic or unresectable melanoma. These patients must have been treated with at least 1 systemic therapy, including PD-1 inhibitors, and if BRAF V600E/K positive, a BRAF inhibitor with or without a MEK inhibitor.3 Lifileucel uses T cells that have been harvested from the tumor tissue, purified and expanded ex vivo in bioreactors, and given back to patients as a living drug.4 The extraction process requires hospitalization and is similar to chimeric antigen receptor T-cell therapy (CAR-T), with an estimated extraction to re-administration time of about 4 to 6 weeks. However, there are several key differences between CAR-T and TIL: CAR-T is blood-derived vs. TIL, which is tumor-derived. CAR-T requires a genetic modification of the product to express a CAR whereas TILs require no engineering. TILs require post-infusion administration of IL-2, and CAR T-cell therapy does not.4
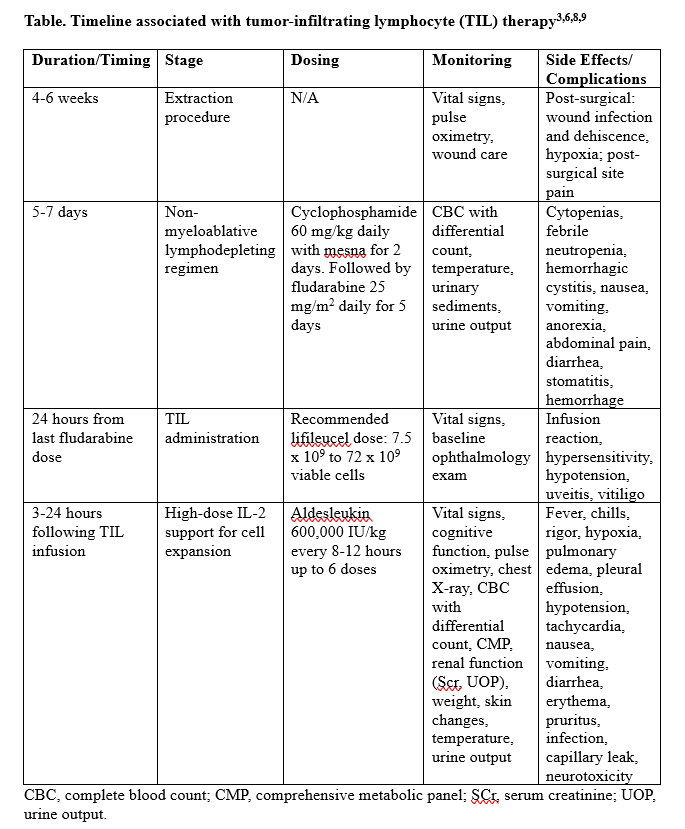
To make room for the new cells, patients must undergo a non-myeloablative lymphodepleting regimen including cyclophosphamide with mesna daily for 2 days followed by fludarabine daily for 5 days. Twenty-four hours following the last fludarabine dose, lifileucel can be given. Three to 24 hours after the completion of lifileucel, high-dose IL-2 infusions are administered to help with cell expansion. This short course of IL-2 boluses is infused every 8 to 12 hours for up to 6 doses.5 An overview of the timeline for tumor-infiltrating lymphocyte therapy may be found in the Table.
Efficacy
In regards to the efficacy of lifileucel, a phase 2, single arm study of heavily pretreated patients with metastatic melanoma (100% had received previous anti–PD-1 or [programmed death ligand 1] PD-L1; 88% had received previous BRAF +/- MEK inhibitor) showed that there was a 36% (24/66) overall response rate (ORR);the median duration of response was not reached at 18.7-months follow-up.6 Long term follow-up has now confirmed patients who were treated with lifileucel maintained their response for up to 5 years. Importantly, the effectiveness of lifileucel remained consistent regardless of prior anti–CTLA-4 therapy, PD-L1 and/or BRAF mutation status. Additionally, lifileucel showed effectiveness among patients who were primary refractory to anti–PD-1/PD-L1 therapy.
In an updated analysis, presented at the Society for Immunotherapy of Cancer Annual Meeting in 2022 and included in its application for FDA approval, an additional 87 patients for a total of 153 patients concluded that the ORR seen in the phase 2 data was confirmed (ORR, 31.4%) and median duration of response was still not reached at a median follow-up of 27.6 months.7,9
Adverse Events (AEs)
Lifileucel was found to have a tolerable safety profile, where the most common AEs were observed during the first 14 days of therapy and were generally transient.8 In the 2022 phase 2 trial, all patients experienced at least 1 AE, with some of the most common grade 3 of 4 AEs being thrombocytopenia (78.2%), anemia (58.2%), and febrile neutropenia (46.8%), aligning with toxicities related to non-myeloablative lymphodepleting treatment and high-dose IL-2.2,9 Lifileucel has warnings for prolonged severe cytopenia, internal organ hemorrhage, severe infection, respiratory failure, cardiopulmonary disorders, and renal impairment.2 There were 6 deaths within 30 days after infusion with 4 related to AEs and 2 to progressive disease; 2 deaths also occurred during lymphodepletion.2,9 The non-myeloablative lymphodepletion regimen caused cytopenias, where granulocyte colony-stimulating factor (G-CSF) can be safely administered the day after the TIL infusion, and drug-induced immunodeficiency requiring antibiotic prophylaxis.8
Toxicities related to cyclophosphamide and fludarabine, such as renal and gastrointestinal toxicities, are to be expected and treated per institutional standard.8 High-dose IL-2 infusion issues can be difficult to manage, and careful monitoring and early treatment are key.8 Related AEs include rigors, fever, hypotension, shortness of breath, pulmonary edema, oliguria, and neurotoxicity.8 Infusion reactions with TIL have been reported in less than 4% of patients and they can be treated with appropriate emergency medications.8 Premedications, such as acetaminophen and diphenhydramine, can be given, but systemic steroids should be avoided in all situations to protect the efficacy of the newly infused TILs.8
Being the first tumor-derived autologous T-cell immunotherapy, lifileucel proved its robust efficacy in heavily pretreated patients and provided a new option for patients whose cancer has progressed on the current standard of care of immune checkpoint inhibitors. TIL therapy continues to make strides, as there is emerging clinical trial data in patients with advanced non-small cell lung cancer who have progressed after nivolumab, treatment-refractory breast cancer in combination with pembrolizumab, and other solid tumors beyond melanoma.5-8

Bethany Pan, PharmD, is a pharmacy resident at UC Davis Health, Sacramento, CA

Ryan Beechinor, PharmD, BCPS, BCOP, is a senior clinical pharmacist at UC Davis Health, Sacramento, and an assistant clinical professor at University of California, San Francisco School of Pharmacy, San Francisco.
References
- Kudchadkar, R.R., Lowe, M. C., Khan, M. K., McBrien, S. M. (2020). Metastatic melanoma. CA: a cancer journal for clinicians. 70(2):78-85. https://doi.org/10.3322/caac.21599
- Amtagvi (lifileucel) [package insert]. Philadelphia, PA: Iovance Biotherapeutics Manufacturing LLC; 2024.
- S. Food and Drug Administration. (February 16, 2024). FDA grants accelerated approval to lifileucel for unresectable or metastatic melanoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-lifileucel-unresectable-or-metastatic-melanoma
- Mullard, A. (2024). Tumour-infiltrating lymphocyte cancer therapy nears FDA finish line. Nature reviews. Drug discovery, 23(1), 3–7. https://doi.org/10.1038/d41573-023-00206-6
- Betof Warner, A., Corrie P. G., Hamid, O. (2023). Tumor-Infiltrating Lymphocyte Therapy in Melanoma: Facts to the Future. Clinical cancer research : an official journal of the American Association for Cancer Research, 29(10):1835-1854. https://doi.org/10.1158/1078-0432.CCR-22-1922
- Sarnaik, A. A., Hamid, O., Khushalani, N. I., Lewis, K. D., Medina, T., Kluger, H. M., Thomas, S. S., Domingo-Musibay, E., Pavlick, A. C., Whitman, E. D., Martin-Algarra, S., Corrie, P., Curti, B. D., Oláh, J., Lutzky, J., Weber, J. S., Larkin, J. M. G., Shi, W., Takamura, T., Jagasia, M., … Chesney, J. A. (2021). Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 39(24), 2656–2666. https://doi.org/10.1200/JCO.21.00612
- Sarnaik, A., Lewis, K., Kluger, H., et al. Lifileucel TIL cell monotherapy in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapy: pooled analysis of consecutive cohorts (C-144-01 study). SITC 37th Annual Meeting (SITC 2022) Abstracts; Boston, MA, November 2022.
- Betof Warner A, Hamid O, Komanduri K, et al. (2024). Expert consensus guidelines on management and best practices for tumor-infiltrating lymphocyte cell therapy. Journal for immunotherapy of cancer, 12(2):e008735. https://doi.org/10.1136/jitc-2023-008735
- Chesney, J., Lewis, K. D., Kluger, H., Hamid, O., Whitman, E., Thomas, S., Wermke, M., Cusnir, M., Domingo-Musibay, E., Phan, G. Q., Kirkwood, J. M., Hassel, J. C., Orloff, M., Larkin, J., Weber, J., Furness, A. J. S., Khushalani, N. I., Medina, T., Egger, M. E., Graf Finckenstein, F., … Sarnaik, A. (2022). Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. Journal for immunotherapy of cancer, 10(12), e005755. https://doi.org/10.1136/jitc-2022-005755