Berkin Kutluk, PharmD Candidate 2024, and Abigail Shockley, PharmD, BCOP
In patients with hormone-receptor (HR)–positive early-stage breast cancer, adjuvant endocrine therapy administered for up to 5 to 10 years significantly reduces recurrence risk and death.1,2 According to the National Comprehensive Cancer Network (NCCN) guidelines, first-line therapy involves tamoxifen or aromatase inhibitors (AIs) in combination with ovarian ablation or suppression (i.e., bilateral oophorectomy or gonadotropin-releasing hormone agonist) in pre-menopausal patients.2 Despite having different side effect profiles, both tamoxifen and AIs contribute to vasomotor symptoms (VMS), such as hot flashes and night sweats.
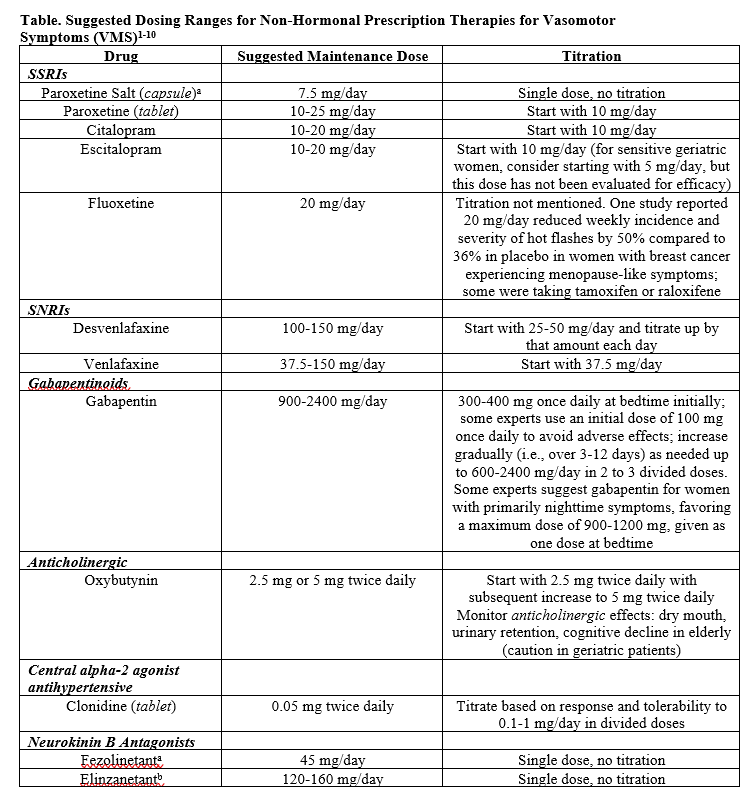
VMS are reported in more than 80% of women receiving tamoxifen or AIs, with hot flashes being the most bothersome symptom.1 Normally, standard of care for VMS is menopausal hormone therapy (MHT) with estrogen-based therapy.3 However, personal history of estrogen-sensitive cancers is an absolute contraindication for patients on endocrine therapy.4 This highlights the importance of non-hormonal therapy options to effectively reduce the frequency and severity of hot flashes in patients with breast cancer (see Table).
- Lambertini, M., Arecco, L., Woodard, T. L., Messelt, A., & Rojas, K. E. (2023). Advances in the Management of Menopausal Symptoms, Fertility Preservation, and Bone Health for Women With Breast Cancer on Endocrine Therapy. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting, 43, e390442. https://doi.org/10.1200/EDBK_390442
- “The 2023 Nonhormone Therapy Position Statement of The North American Menopause Society” Advisory Panel (2023). The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause (New York, N.Y.), 30(6), 573–590. DOI:10.1097/GME.0000000000002200
- Simon, J. A., Anderson, R. A., Ballantyne, E., Bolognese, J., Caetano, C., Joffe, H., Kerr, M., Panay, N., Seitz, C., Seymore, S., Trower, M., Zuurman, L., & Pawsey, S. (2023). Efficacy and safety of elinzanetant, a selective neurokinin-1,3 receptor antagonist for vasomotor symptoms: a dose-finding clinical trial (SWITCH-1). Menopause (New York, N.Y.), 30(3), 239–246. doi: 10.1097/GME.0000000000002138.
- US Pharmacist. (2018, December 19). Oxybutynin could help hot flashes when hormones not indicated. Retrieved January 5, 2024 from https://www.uspharmacist.com/article/oxybutynin-could-help-hot-flashes-when-hormones-not-indicated
- Loprinzi, C. L., Sloan, J. A., Perez, E. A., Quella, S. K., Stella, P. J., Mailliard, J. A., Halyard, M. Y., Pruthi, S., Novotny, P. J., & Rummans, T. A. (2002). Phase III evaluation of fluoxetine for treatment of hot flashes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 20(6), 1578–1583. https://doi-org.ezproxy.lib.utexas.edu/10.1200/JCO.2002.20.6.1578
- Khan, S. J., Kapoor, E., Faubion, S. S., & Kling, J. M. (2023). Vasomotor Symptoms During Menopause: A Practical Guide on Current Treatments and Future Perspectives. International journal of women’s health, 15, 273–287. https://doi.org/10.2147/IJWH.S365808
- Shaukat, A., Mujeeb, A., Shahnoor, S., Nasser, N., & Khan, A. M. (2023). Veozah (Fezolinetant): A Promising Non-Hormonal Treatment for Vasomotor Symptoms in Menopause. Health science reports, 6(10), e1610. https://doi.org/10.1002/hsr2.1610
- Lexicomp Online, Lexi-Drugs Online. Waltham, MA: UpToDate, Inc.; July 30, 2021. Retrieved January 5, 2024 from https://online.lexi.com.
- Lexicomp Online, Lexi-Drugs Online. Waltham, MA: UpToDate, Inc.; July 30, 2021. Retrieved January 5, 2024 from https://online.lexi.com.
- Clinical Pharmacology powered by ClinicalKey. Philadelphia (PA): Elsevier. Copyright 2024. Retrieved March 5, 2024 from http://www-clinicalkey-com.ezproxy.lib.utexas.edu (subscription required to view).
Non-pharmacological and pharmaceutical options for VMS
In managing VMS, the NCCN guidelines incorporate a range of interventions, including non-pharmacological approaches, such as cognitive-behavioral therapy, acupuncture, clinical hypnosis, and weight loss. Among pharmaceutical options, venlafaxine, a serotonin-norepinephrine reuptake inhibitor, stands out, with Loprinzi et al. demonstrating reductions of 37% and 61% in median hot flash scores at 4 weeks for 37.5 mg and 75 mg doses respectively compared to 27% in the placebo group.5 Another randomized placebo-controlled trial by Boekhout et al. compared clonidine 0.1 mg to venlafaxine 75 mg, revealing lower average daily hot flash scores at 12 weeks, while venlafaxine provided more immediate relief.6 Although in this study clonidine proved to be a potential option, prescribers must take caution, as clonidine may induce dry mouth, constipation, and hypotension.
Selective serotonin reuptake inhibitors (SSRIs) like citalopram, paroxetine, and fluoxetine have also demonstrated positive effects in randomized placebo-controlled trials.3 However, paroxetine and fluoxetine do have potential interactions with tamoxifen.2,4 Additionally, gabapentin and pregabalin have efficacy, but both medications require careful dose escalation due to sedation.
Another option, oxybutynin, though effective in reducing hot flashes, presents anticholinergic effects, such as dry mouth and cognitive decline in the elderly.1,4 However, there is anecdotal evidence suggesting oxybutynin could be effective in the treatment of hot flashes refractory to gabapentin and venlafaxine.7 Of all these, low-dose paroxetine is the only FDA-approved non-hormone treatment for moderate-to-severe VMS.8
The unmet need for safe and effective non-hormonal therapy for VMS relief in women with menopause who are unwilling or unable to take hormone therapy led to a burgeoning interest in neurokinin B antagonists. Fezolinetant [Veozah, Astellas] is a novel FDA-approved neurokinin B receptor-3 antagonist for treatment of moderate-to-severe VMS due to menopause. The pathophysiology of VMS involves a disruption in the hypothalamic thermoregulatory zone.9 Declining estrogen in menopause leads to hypersecretion of neurokinin B from kisspeptin, neurokinin B, and dynorphin (KNDy) neurons, causing hot flashes. Fezolinetant works by targeting the hypothalamic neurons and decreasing neurokinin B binding to reduce hot flashes.
Fezolinetant clinical trials
Two phase 3 clinical trials (SKYLIGHT 1 and 2) demonstrated the safety and efficacy of fezolinetant in non-hormonal VMS treatment-naïve patients.10,11 In the first study, SKYLIGHT 1, participants were randomly assigned to 30 mg or 45 mg fezolinetant daily for 12 weeks. Both doses significantly reduced the frequency and severity of VMS at weeks 4 and 12. By week 12, frequency of VMS was reduced by at least 50% in 77 of 173 (45%) participants in the 30 mg group and 99 of 174 (57%) participants in the 45 mg group, versus 52 of 175 (30%) participants in the placebo group.
Rare cases of elevated liver function tests (LFTs) were reported. This prompted FDA to include a warning for testing liver function and liver injury prior to taking fezolinetant. Patients exhibiting symptoms of liver damage and taking CYP1A2 inhibitors should avoid the drug.12 The most common side effects were headache, followed by abdominal pain, diarrhea, and insomnia. The second double-blind placebo-controlled study, SKYLIGHT 2, showed similar results in women with moderate-to-severe VMS. Fezolinetant appears to be safe and effective in reducing VMS by over 50% from baseline.11
Conclusion
In conclusion, venlafaxine, clonidine, SSRIs, gabapentin, pregabalin, and oxybutynin are effective non-hormonal therapies. However, there is evidence supporting fezolinetant as a potential novel first-in-class non-hormonal neurokinin B antagonist for VMS relief. Fezolinetant’s clinically meaningful improvement in menopause-specific quality of life and FDA approval have the potential to impact the current guidelines for management of VMS in patients with breast cancer receiving anti-estrogen therapy. Because fezolinetant was not studied in patients with breast cancer experiencing menopause-like symptoms and only studied in menopausal women, future research should be conducted to evaluate the safety and efficacy of this novel therapeutic option. Despite the lack of evidence in this specific patient population, fezolinetant remains a possible agent for patients with breast cancer experiencing refractory VMS due to anti-estrogen therapy.

Berkin Kutluk, PharmD Candidate 2024, is a student at The University of Texas at Austin College of Pharmacy. He is to general PGY1 residency programs offering a diverse set of rotations and experiences to help determine what to specialize in PGY2 and beyond.

Abigail Shockley, PharmD, BCOP, is a clinical pharmacy specialist in breast medical oncology at MD Anderson Cancer Center in Houston, TX.
References
- Lambertini, M., Arecco, L., Woodard, T. L., Messelt, A., & Rojas, K. E. (2023). Advances in the Management of Menopausal Symptoms, Fertility Preservation, and Bone Health for Women With Breast Cancer on Endocrine Therapy. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting, 43, e390442. https://doi.org/10.1200/EDBK_390442
- Gradishar, W. J., Moran, M. S., Abraham, J., Abramson, V., Aft, R., Agnese, D., Allison, K. H., Anderson, B., Burstein, H. J., Chew, H., Dang, C., Elias, A. D., Giordano, S. H., Goetz, M. P., Goldstein, L. J., Hurvitz, S. A., Jankowitz, R. C., Javid, S. H., Krishnamurthy, J., Leitch, A. M., … Kumar, R. (2023). NCCN Guidelines® Insights: Breast Cancer, Version 1.2024. National Comprehensive Cancer Network (NCCN). Retrieved January 5, 2024 from https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- “The 2022 Hormone Therapy Position Statement of The North American Menopause Society” Advisory Panel (2022). The 2022 hormone therapy position statement of The North American Menopause Society. Menopause (New York, N.Y.), 29(7), 767–794. DOI:10.1097/GME.0000000000002028
- “The 2023 Nonhormone Therapy Position Statement of The North American Menopause Society” Advisory Panel (2023). The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause (New York, N.Y.), 30(6), 573–590. DOI:10.1097/GME.0000000000002200
- Loprinzi, C. L., Kugler, J. W., Sloan, J. A., Mailliard, J. A., LaVasseur, B. I., Barton, D. L., Novotny, P. J., Dakhil, S. R., Rodger, K., Rummans, T. A., & Christensen, B. J. (2000). Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet (London, England), 356(9247), 2059–2063. doi: 10.1016/S0140-6736(00)03403-6.
- Boekhout, A. H., Vincent, A. D., Dalesio, O. B., van den Bosch, J., Foekema-Töns, J. H., Adriaansz, S., Sprangers, S., Nuijen, B., Beijnen, J. H., & Schellens, J. H. (2011). Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 29(29), 3862–3868. doi: 10.1200/JCO.2010.33.1298.
- Leon-Ferre, R. A., Novotny, P. J., Wolfe, E. G., Faubion, S. S., Ruddy, K. J., Flora, D., Dakhil, C. S. R., Rowland, K. M., Graham, M. L., Le-Lindqwister, N., Smith, T. J., & Loprinzi, C. L. (2019). Oxybutynin vs Placebo for Hot Flashes in Women With or Without Breast Cancer: A Randomized, Double-Blind Clinical Trial (ACCRU SC-1603). JNCI cancer spectrum, 4(1), pkz088. https://doi.org/10.1093/jncics/pkz088
- Khan, S. J., Kapoor, E., Faubion, S. S., & Kling, J. M. (2023). Vasomotor Symptoms During Menopause: A Practical Guide on Current Treatments and Future Perspectives. International journal of women’s health, 15, 273–287. https://doi.org/10.2147/IJWH.S365808.
- Pinkerton, J. V., Redick, D. L., Homewood, L. N., & Kaunitz, A. M. (2023). Neurokinin Receptor Antagonist, Fezolinetant, for Treatment of Menopausal Vasomotor Symptoms. The Journal of clinical endocrinology and metabolism, 108(11), e1448–e1449. doi: 10.1210/clinem/dgad209.
- Lederman, S., Ottery, F. D., Cano, A., Santoro, N., Shapiro, M., Stute, P., Thurston, R. C., English, M., Franklin, C., Lee, M., & Neal-Perry, G. (2023). Fezolinetant for treatment of moderate-to-severe vasomotor symptoms associated with menopause (SKYLIGHT 1): a phase 3 randomised controlled study. Lancet (London, England), 401(10382), 1091–1102. doi: 10.1016/S0140-6736(23)00085-5.
- Johnson, K. A., Martin, N., Nappi, R. E., Neal-Perry, G., Shapiro, M., Stute, P., Thurston, R. C., Wolfman, W., English, M., Franklin, C., Lee, M., & Santoro, N. (2023). Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated With Menopause: A Phase 3 RCT. (SKYLIGHT 2) The Journal of clinical endocrinology and metabolism, 108(8), 1981–1997. doi: 10.1210/clinem/dgad058.
- Grant, A. (2023, May 12). FDA approves novel drug to treat moderate to severe hot flashes caused by menopause. U.S. Food and Drug Administration. Retrieved January 5, 2024 from https://www.fda.gov/news-events/press-announcements/fda-approves-novel-drug-treat-moderate-severe-hot-flashes-caused-menopause