Christian Watts Pharm.D., MSHI Candidate, Larry Buie Pharm.D., BCOP, FCCP, FASHP, Beth Sandy, MSN, CRNP | Dec 13, 2021 12:27:20 PM
Introduction: Epidermal growth factor receptor (EGFR) in non-small cell lung cancer (NSCLC) typically harbors the classical mutations of EXON 19 deletion or EXON 21point mutation L858R. However, less commonly, EGFR mutations can be exhibited as an EXON 20 insertion (EGFRex20ins), which are generally resistant to traditional EGFR therapies. In 2021, the first treatment for EGFRex20ins treatment was amivantamab (RYBREVANT™), a monoclonal antibody approved for treatment after disease progression with platinum-based chemotherapy. More recently in 2021, a new oral therapy, mobocertinib, has also been approved for EGFRex20ins.
Indication: Mobocertinib (EXKIVITY™) is the first FDA approved oral tyrosine kinase treatment for patients with locally advanced or metastatic NSCLC containing the EGFRex20ins. Mobocertinib is approved specifically for patients with an EGFRex20ins mutation, as confirmed by an FDA approved test, whose disease progressed during or after platinum-based chemotherapy. The FDA granted accelerated approval with breakthrough therapy and orphan drug designations to mobocertinib on September 15th, 2021, after a phase 1/2 study showed favorable overall response rates (ORR) and duration of response (DoR).1 Confirmatory trials are required for continued FDA approval for this indication and are ongoing.
Specific Mutation: EGFRex20ins mutations are relatively rare, occurring in only 4-12% of EGFR mutations and 2% of NSCLCs1. The mutation is characterized as an in-frame insertion or duplications of 3 to 21 base pairs, specifically within the amino acid positions 762 and 774 of the EGFR protein.2 The exon 20 insertion mutation leads to increased cell survival and proliferation promoting the spread of cancerous cells. The EGFRex20ins insertion mutation has been linked to less favorable outcomes and response to other current EGFR directed therapies such as erlotinib and afatanib are poor. Therefore, mobocertinib provides an advantage for EGFRex20ins-positive NSCLC that has not been seen by any other oral therapies.
Efficacy: The efficacy of mobocertinib was assessed in a study of 114 patients with EGFRex20ins-positive locally advanced or metastatic NSCLC, who received prior platinum-based chemotherapy. The study was shown to have an overall response rate of 28% (95% CI: 20%-37%) per the independent review committee (IRC) with a median duration of response of 17.5 months.1 The median overall and progression free survival were 24 months and 7.3 months, respectively.1
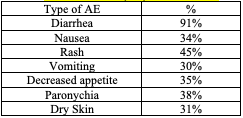
Adverse Events: The most common adverse reactions (>20%) are seen in Table 1. 3
Table 1. Adverse Events (AE) Mobocertinib1
Educate patients to report symptoms of syncope, light headedness, palpitations, chest pain, dizziness, shortness of breath, or cough, to their oncology team. Mobocertinib can cause life threating heart problems such as QTc prolongation (11%), torsades de pointes (1.2%), heart failure (2.7%), and interstitial lung disease/pneumonitis (4.3%)3.
Dosing and Administration: The recommended dosing for Mobocertinib is 160mg orally daily, with or without food, until disease progression or intolerable toxicity. Mobocertinib comes in 40mg capsule formulations and patients are instructed to take 4 capsules at the same time each day. Adverse events may result in withholding, reducing, or discontinuing mobocertinib therapy based on severity If a patient thinks they forgot to take their dose or they think they have vomited the medication, advise them not to take another dose, but to wait until the next day and resume regular dose.
Drug Interactions: Mobocertinib is a substrate of CYP 3A. Concomitant use with CYP 3A inhibitors can increase Mobocertinib plasma concentrations. This increases the risk for adverse events. Coadministration with CYP3A inhibitors should be avoided and when not possible, consider reducing the mobocertinib dose by 50%.3 Concomitant use with CYP3A inducers should be avoided due to anti-tumor activity reduction. Eating grapefruit or drinking grapefruit juice should be avoided due to the risk of Mobocertinib concentration in the blood being increased.3
Patient Support: Advanced practice providers are involved with ensuring medication access, prior authorization, and copay assistance awareness. They also play a key role in educating patients about the possible adverse effects and drug interactions associated with mobocertinib, particularly the most common adverse events mentioned in Table 1.
Oncology providers should review the idea of taking 4 pills daily, with or without food, not only with patients but also their caregivers. Pillboxes, alarms, calendars and other tools can be used to improve adherence. Patients should also be reminded to report any adverse events that occur to their oncology care team.
Finally, remember to bring the patient back in to review labs and compliance. Usually, follow-up in 2-3 weeks is a good time to reinforce the medication dose, frequency, and assess for any side effects that the patient may have. After that, it is up to the oncology providers to determine what intervals are appropriate for scanning and symptom follow up appointments.
References:
1. Zhou C, Ramalingam SS, Kim TM, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer. A phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. Published online October 14, 2021. doi:1001/jamaoncol.2021.4761
2. Vyse, S. and Huang, P., 2019. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduction and Targeted Therapy, [online] 4(1). https doi.org/:// 10.1038/s41392-019-0038-9.com.
3. 2021. EXKIVITY™ (mobocertinib) Package Insert. [online] Available at: <https://www.exkivity.com/.> [Accessed 2 December 2021].

Christian Watts, SIUE School of Pharmacy, Edwardsville, IL

Beth Sandy, Abramson Cancer Center, University of Pennsylvania
W. Buie, Memorial Sloan Kettering Cancer Center, New York, NY