Sijimol Mathew, DNP, APRN
The patient with cancer has many risk factors for developing lactic acidosis. The diagnosis of cancer itself contributes to the overproduction of lactate caused by thiamine and or riboflavin deficiencies and embolization of the microvasculature by malignant cells.1 The presence of tumor cells decreases their ability to clear lactate from the liver. Neutropenic patients are at high risk for sepsis and impaired tissue perfusion leads to lactic acidosis. Understanding the pathophysiology and eliminating the etiology is the key to management.
Case Presentation
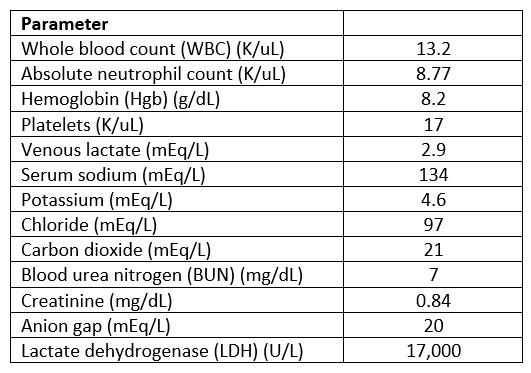
Mr. A is a 61-year-old male with large B-cell lymphoma with central nervous system disease who has received multiple lines of therapy including stem cell transplant. He is currently being treated with fractionated cyclophosphamide with dexamethasone and rituximab and concurrent brain radiation, and he was admitted for chimeric antigen receptor (CAR) T-cell therapy. See Table 1 for Mr. A’s laboratory values at the beginning of this episode.
Table 1. Admission Laboratory Values
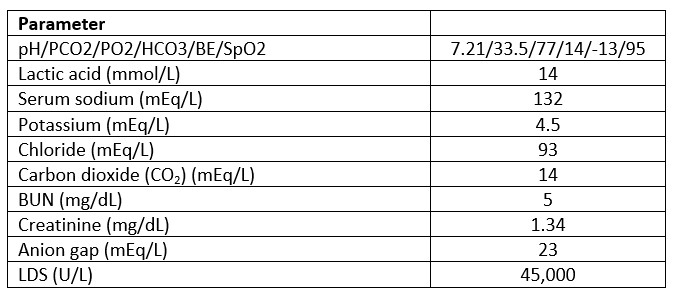
The next day the patient was noted to have tachycardia and tachypnea followed by hypotension. The patient had some response with fluid resuscitation. Sepsis works-up was completed and the patient was started on broad-spectrum antibiotics. However, that night the clinical emergency response team was called for hypotension and tachypnea, and the patient required transfer to the intensive care unit for treatment with vasopressors. Arterial blood gases (ABG) showed acidosis. See Table 2 for laboratory values upon transfer to the intensive care unit.
Table 2. Intensive Care Unit Laboratory Values
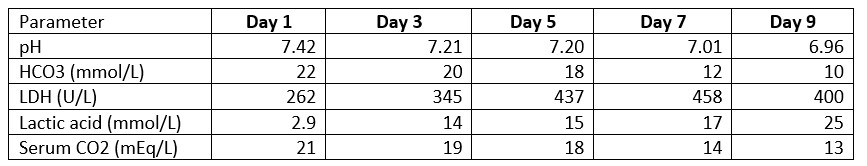
Nephrology was consulted. Despite the resuscitation attempts, patient continued to have elevated lactic acid levels and multiple vasopressors needing continuous renal replacement therapy (CRRT) and mechanical ventilation. The patient’s liver function tests were elevated, and his blood glucose levels were noted to be as low as 40 mg/dl. An intravenous sodium bicarbonate drip and dextrose 50% infusion were initiated. He also received a thiamine injection. When there was no improvement in the patient’s acidosis over the next few days (see Table 3), the family opted for comfort care measures.
Table 3. Timeline of Laboratory Blood Values
Causes of Lactic Acidosis
Metabolic acidosis with lactate levels greater than 5 mmol/L and pH less than 7.35 is defined as lactic acidosis.2 Lactic acid is transported by the blood, primarily to the liver and kidneys as well as the heart and other tissues. The conversion of lactic acid back to glucose in the liver is known as the Cori cycle.3 The Cori cycle normally maintains the balance between the production and use of lactic acid.
The pathophysiology of lactic acidosis can be explained as follows. Type A lactic acidosis is due to tissue hypoperfusion, such as sepsis, cardiac failure, hypovolemia, or cardiopulmonary arrest.4 Type B lactic acidosis includes toxin-induced impairment of cellular metabolism without evidence of systemic hypoperfusion. It is associated with liver failure, malignancies, and certain drugs (biguanides).5 While lactic acidosis occurs rarely in patients with leukemia or lymphoma, 85% of cases occur in patients with hematological malignancies, and 15% occur in patients with solid tumor malignancies.1 Lactic acidosis occurs in malignancies due to the lack of perfusion from tumor burden and liver dysfunction from metastatic lesions in the hepatic parenchyma. The Warburg effect is evident while fulfilling the tumor cell needs with high glucose intake, and lactic acid is released. Tumor tissues show on average a 10-fold higher rate of glucose uptake and glycolysis compared to healthy tissues.2
Early Detection and Management
Tachypnea, tachycardia, altered mental status, nausea, cool clammy extremities, decreased urine output and muscle weakness are the clinical manifestations of lactic acidosis. The brain sends a signal to increase breathing when high acid levels are detected in the blood.
Severe acidosis leads to a reduction in systemic blood pressure from reduced cardiac contractility and vascular hypo responsiveness.
Monitor patients’ blood sugar and serum CO2 levels in the daily chemistries. Hypoglycemia and acidosis require a search for quick diagnosis and management. A concomitant decrease in serum bicarbonate level occurs with the severity of lactic acid elevation.
The atypical presentation makes lactic acidosis difficult to diagnose and the complexity limits the treatment options. It is associated with high mortality and poor outcome.4 Prompt identification is important because once a patient starts declining treatment options are limited.
Regardless of the mechanism, removal of the tumor (by chemotherapy, irradiation, or surgery) usually corrects the lactic acidosis.
Monitoring the electrolytes and anion gap may help to identify the cause of acidosis.
Blood gas values determine the blood pH, PCO2 and O2 levels. A venous pH may be adequate unless the respiratory disorder is present or to determine the degree of respiratory compensation for metabolic disorders.
The clinical impact of bicarbonate therapy on acidosis is controversial. It is initiated only in acute metabolic acidosis with severe acidemia (pH less than 7.1) to reduce myocardial depression and arrhythmias.6
Mechanical ventilation and renal replacement therapy are cornerstones in managing critically ill patients with severe acidemia.
Conclusion
Early recognition of lactic acidosis, the inciting etiology, and prompt treatment are crucial in successfully reversing lactic acidosis, thereby preventing the fatal outcome of this rare oncological emergency.
Sijimol Mathew DNP, APRN, is an advanced practice provider at the Nocturnal Program at UT MD Anderson Cancer Center, Houston, Texas.
References
- Wahab, A., Kesari, K., J. Smith, S., Liu, Y., & Barta, S. K. (2018). Type B lactic acidosis, an uncommon paraneoplastic syndrome. Cancer Biology & Therapy, 19(2), 101–104. https://doi.org/10.1080/15384047.2017.1394550
- Sanivarapu, R., Upadrista, P. K., Otero-Colon, J., Shah, K., Cadet, B., Tao, Q., & Iqbal, J. (2022). An Oncological Emergency: Severe Type B Lactic Acidosis From Warburg Effect in Diffuse Large B-cell Lymphoma. Cureus. https://doi.org/10.7759/cureus.26557
- Townsend, C. (2022). Cori Cycle. Science Direct.
- Wang, C., Lv, Z., & Zhang, Y. (2022). Type B lactic acidosis associated with diffuse large B-cell lymphoma and the Warburg effect. Journal of International Medical Research, 50(1), 030006052110677. https://doi.org/10.1177/03000605211067749
- Pattharanitima, P., Thongprayoon, C., Petnak, T., Srivali, N., Gembillo, G., Kaewput, W., Chesdachai, S., Vallabhajosyula, S., O’Corragain, O. A., Mao, M. A., Garovic, V. D., Qureshi, F., Dillon, J. J., & Cheungpasitporn, W. (2021). Machine Learning Consensus Clustering Approach for Patients with Lactic Acidosis in Intensive Care Units. Journal of Personalized Medicine, 11(11), 1132. https://doi.org/10.3390/jpm11111132
- Jaber, S., Paugam, C., Futier, E., Lefrant, J. Y., Lasocki, S., Lescot, T., Pottecher, J., Demoule, A., Ferrandière, M., Asehnoune, K., Dellamonica, J., Velly, L., Abback, P. S., de Jong, A., Brunot, V., Belafia, F., Roquilly, A., Chanques, G., Muller, L., . . . Kipnis, E. (2018). Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. The Lancet, 392(10141), 31–40. https://doi.org/10.1016/s0140-6736(18)31080-8

Nocturnal Program APP
UT MD Anderson Cancer Center,
Houston, Texas