Kathryn Green, MSN, ACNP-BC
Hepatocellular cancer (HCC) is a common cancer globally, and there are more than 40,000 new cases yearly with an estimated 29,380 deaths per year in the United States.1 The 5-year relative survival rate is 21%, and it is lower for patients with unresectable disease.1 A new treatment regimen of durvalumab [Imfinzi, AstraZeneca] and tremelimumab [Imjudo, AstraZeneca] was approved in October 2022 by the FDA for adults with unresectable HCC based on the results of the HIMALAYA trial.2,3 This regimen provides an additional treatment option for patients with HCC.
Combination immunotherapy, which targets different steps of the immune response, has shown improved tumor control in many solid tumors.4 Durvalumab is a programmed death ligand 1 (PD-L1) inhibitor and tremelimumab is a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor.2,3 The HIMALAYA trial was a global phase 3 trial of 1171 patients with unresectable HCC who had not received prior systemic therapy.5 The primary endpoint of the trial was overall survival (OS) with durvalumab and tremelimumab compared with sorafenib.5 Patients were randomly assigned to receive tremelimumab (300 mg, one dose) plus durvalumab (1500 mg every 4 weeks), single-agent durvalumab (1500 mg every 4 weeks), or sorafenib (400 mg twice daily).5 The dosing format of Single-dose Tremelimumab and Regular-Interval Durvalumab was given the acronym STRIDE.5 The dosing is described in the Figure.
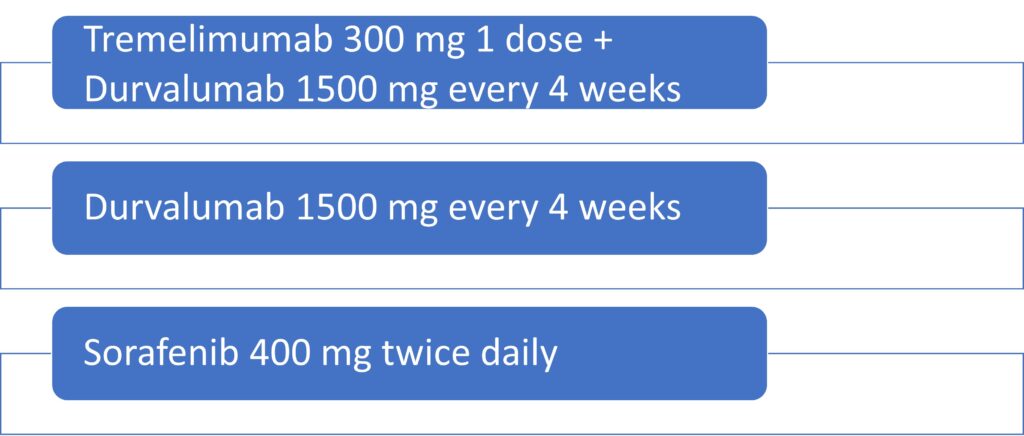
Figure. STRIDE Trial Dosing
Baseline characteristics
The inclusion and exclusion criteria are summarized in Table 1. Adult patients were selected from 16 countries, with an average age of 64 years and age range from 18 to 88 years.5 Eighty-four percent of patients were men.5 About 25% of patients had microvascular invasion and a little over half had extrahepatic spread.5 One hundred seventeen patients had prior disease related radiotherapy.5
| Table 1. HIMALAYA Inclusion and Exclusion Criteria5 |
| Inclusion Criteria |
| – Patients were required to have unresectable disease, no previous systemic therapy, and be ineligible for locoregional therapy. – Patients needed to have an Eastern Cooperative Oncology Group (ECOG) performance score of 0-1. – Patients were required to have adequate liver function (Child-Pugh class A or B, and Barcelona Clinic Liver Cancer [BCLC] stage B or C). – Patients with a history of hepatitis B or C, and those with non-viral etiologies were also included.5 |
| Exclusion Criteria |
| – Patients with co-infection of hepatitis B and C viruses were excluded. – Patients with ascites and hepatic encephalopathy were excluded. |
Outcomes
The median OS for STRIDE was 16.43 months compared to 13.77 months for sorafenib.5 Survival rates for STRIDE and sorafenib were 48.7% and 41.5% at 18 months, 40.5% and 32.6% at 24 months, and 30.7% and 20.2% at 36 months.5 Patients in the STRIDE arm had a 20.1% objective response rate (ORR) and a complete response (CR) rate of 3.1%.5 Comparatively, sorafenib patients had a 5.1% ORR and no patients had a CR.5 The median duration of response was 22.3 months for STRIDE and 18.4 months for sorafenib.5 Participants reported outcome measures during the trial to identify time to deterioration of quality of life. STRIDE patients reported a median time to deterioration of 7.5 months and sorafenib patients reported 5.7 months.5
Adverse events
Adverse events in the STRIDE trial are detailed in Table 2. Notable immune-mediated adverse effects (IMAE) of the STRIDE regimen include pneumonitis, endocrinopathies, nephritis, pancreatitis, hepatitis, dermatitis, and colitis.5 The most common IMAEs were diarrhea and colitis, dermatitis and rash, and elevation in liver enzymes and bilirubin.5
| Table 2. STRIDE vs Sorafenib: Adverse Events (AEs)5 | ||
| STRIDE | Sorafenib | |
| TRAEs | 75.8% | 84.8% |
| Grade 3 and 4 AEs | 50.5% | 52.4% |
| Discontinuation | 13.7% | 16.8% |
| Required high-dose corticosteroids | 20.1% | 1.6% |
| IMAEs leading to discontinuation | 5.7% | 1.6% |
| STRIDE, Single-dose Tremelimumab and Regular-Interval Durvalumab; TRAEs, treatment-related adverse events; IMAEs, immune-mediated adverse events. | ||
Dosing and administration
STRIDE is standard dosing of tremelimumab (300 mg, one dose) plus durvalumab (1500 mg every 4 weeks).2,3,5 Tremelimumab is infused over 60 minutes, and patients should be observed for 60 minutes before starting durvalumab.2,3 Durvalumab is infused over 60 minutes.2,3 Subsequent durvalumab infusions are given over 60 minutes and do not require an observation period.2, 3 Therapy can continue until progression or unacceptable toxicity. Infusion reactions are possible and were seen in 2.6% of patients in the HIMALAYA trial.2,3,5 Providers should manage infusion reactions per institutional protocol and can consider premedicating patients before future infusions depending on the severity of the reaction.2,3
There are no recommended dose reductions for AEs, but the prescribing information recommends holding therapy for most Grade 2 and 3 reactions and treating with corticosteroids slowly tapered once symptoms improve or resolve.2,3 Providers should permanently discontinue therapy for patients with Grade 4 reactions.2,3 More specific recommendations based on individual IMAEs are listed in the prescribing information.
Considerations for oncology APPs
Based on the results of the HIMALAYA trial, the combination regimen of tremelimumab-actl and durvalumab with STRIDE dosing was added to the National Comprehensive Cancer Network (NCCN®) Clinical Practice Guidelines in Oncology as a category 1 recommendation for first-line systemic therapy of patients with unresectable HCC.6 The combination of atezolizumab [Tecentriq, Genentech] and bevacizumab [Avastin, Genentech] is another NCCN category 1 recommendation for first-line systemic therapy for this population but requires endoscopic assessment for varices prior to starting treatment and is only approved for Child-Pugh Class A liver function.6 When choosing between regimens for HCC, the APP should consider if patients can wait for additional testing such as endoscopy to start therapy. Liver function can decompensate rapidly in this population, so the ability to start treatment sooner can improve outcomes. STRIDE dosing is approved for patients with Child-Pugh Class A and B liver function, so can be used safely in more patients than atezolizumab and bevacizumab. The approval of the combination of tremelimumab-actl and durvalumab provides an additional treatment option for patients with unresectable HCC.

References
- Siegel, R. L., Miller, K. D., Wagle, N. S, & Jemal, A. (2023). Cancer statistics, 2023. CA: a cancer journal for clinicians, 73(1),17-48.
- Imfinzi (durvalumab) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals US; November 2022.
- Imjudo (tremelimumab-actl) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals US; November 2022.
- Lu, L., Zhan, M., Li, X., Zhang, H., Dauphars, D.J., Jiang, J., Yin, H., Li, S., Luo, S., Li, Y., and He, Y. (2022). Clinically approved combination immunotherapy: Current status, limitations, and future perspective. Current research in immunology, 3, 118-127.
- Abou-Alfa, G. K., Lau, G., Kudo, M., Chan, S. L., Kelley, R. K., Furuse, J., Sukeepaisarnjaroen, W., & HIMAMALYA investigators. (2022). Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence, 1(8), 1-12. https://evidence.nejm.org/doi/full/10.1056/EVIDoa2100070
- NCCN clinical practice guidelines in oncology Hepatocellular Carcinoma version 1.2023. https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf