Jina Yun, PharmD
Breast cancer is the most prevalent cancer in women in the United States, affecting 1 out of 8 women.1 It is the second leading cause of cancer-related deaths, and patients with distant metastatic disease have a 5-year relative survival rate of approximately 30%.1,2 For patients with locally advanced or metastatic hormone receptor positive (HR+)/human epidermal growth factor 2 negative (HER2-)disease, the first-line standard of care (SOC) treatment includes a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor with either an aromatase inhibitor (AI) or fulvestrant.1 Nearly half of patients who progress on these therapies develop a mutation in estrogen receptor 1 (ESR1), which is a known resistance mechanism to AIs.3 While many patients eventually become resistant to endocrine therapies, the options following progression have not been well established.
Elacestrant (Orserdu, Stemline Therapeutics) is a novel therapy that has been approved by the Food and Drug Administration (FDA) for the treatment of postmenopausal women or adult men with ER+, HER2-, ESR1-mutated advanced or metastatic breast cancer who have progressed on at least one line of endocrine therapy. Elacestrant is an oral selective ER antagonist that halts the 17-estradiol mediated cell proliferation pathway, ultimately leading to inhibition of tumor growth.4 Information regarding elacestrant is summarized in Table 1. Of note, the FDA only has one approved diagnostic device for the ESR1 mutation, which may be important to consider for financial coverage of mutation testing.5
Table 1. Elacestrant Drug Information4
| Indication | Treatment of postmenopausal women or adult men with estrogen receptor (ER)-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer who have progressed on at least one line of endocrine therapy |
| Dose | 345 mg by mouth once daily |
| Adverse Drug Event (ADE) Dose Adjustment | First dose reduction: 258 mg by mouth once dailySecond dose reduction: 172 mg by mouth once daily |
| Tablet size | 345 mg tablet86 mg tablet |
| Administration | Take with foodIf dose is missed more than 6 hours (or vomited), skip the dose and take next dose at scheduled time |
| Notable Drug Interactions | Avoid concurrent use with moderate or strong CYP3A4 inhibitors or inducersReduce the dose of P-gp substrate when used concurrently with elacestrant |
EMERALD Trial
The approval of elacestrant is based on the results of the EMERALD trial. This was an international, multicenter, randomized, open-label, phase III trial that examined postmenopausal women or adult men with ER+/HER- recurrent or metastatic breast cancer who have progressed on one or two prior lines of endocrine therapy. Patients with metastatic visceral disease, relevant cardiovascular events or cerebrovascular events within 6 months of enrollment of the trial were specifically excluded. Patients were randomized as shown in the Figure. Of note, the elacestrant doses used in the EMERALD trial were higher than the FDA-recommended doses for this indication.
Figure. Randomization of patients in the EMERALD Trial6
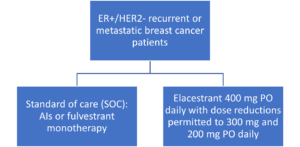
Baseline Characteristics
The trial included 477 patients who received the assigned interventions. The median age was 63 years old with 97.5% of patients being female and 88.4% being Caucasian. Fifty four percent of patients had only one prior line of endocrine therapy and 79.9% had no prior lines of chemotherapy for recurrent or metastatic disease. Most patients had received an AI as the prior endocrine therapy. Notably, 47.8% of patients had an ESR1 mutation.6
Efficacy Outcomes
At the 6-month follow up, the progression free survival (PFS) rates were 34.3% in the elacestrant arm and 20.4% in the SOC arm. This difference was more pronounced when patients with ESR1 mutations were specifically observed (40.8% vs. 19.1%). The 12-month PFS results were also better with the elacestrant group when looking at all patients (22.3% vs. 9.4%) as well as patients with ESR1 mutations (26.8% vs. 8.2%). At this time, the trial does not have fully published overall survival (OS) results.6
Safety Outcomes
Of patients receiving elacestrant, 90.2% experienced an adverse drug event (ADE) while 86% experienced an ADE in the standard of care arm. The most commonly observed ADEs and laboratory changes are listed in Table 2. In addition, 6.3% of patients receiving elacestrant required treatment discontinuation, which was similar to the SOC group of 4.4%. In the elacestrant group, 3% of patients required a dose reduction due to ADEs while no patients in the SOC group required a dose reduction.6
Table 2. Most Common ADEs and Laboratory Changes in the EMERALD Trial4,6
| Elacestrant Group (%) | SOC Group (%) | |||
| All Grades | Grade 3-4 | All Grades | Grade 3-4 | |
| ADEs | ||||
| Nausea | 35 | 2.5 | 18.8 | 0.9 |
| Fatigue | 19 | 0.8 | 18.8 | 0.9 |
| Vomiting | 19 | 0.8 | 8.3 | 0 |
| Decreased Appetite | 14.8 | 0.8 | 9.2 | 0.4 |
| Arthralgia | 14.3 | 0.8 | 16.2 | 0 |
| Diarrhea | 13.9 | 0 | 10 | 0.9 |
| Back pain | 13.9 | 2.5 | 9.6 | 0.4 |
| Headache | 12.2 | 1.7 | 11.4 | 0 |
| Constipation | 12.2 | 0 | 6.6 | 0 |
| Hot flush | 11.4 | 0 | 8.3 | 0 |
| Dyspepsia | 10.1 | 0 | 2.6 | 0 |
| Laboratory Changes | ||||
| Cholesterol increase | 30 | 1 | 17 | 0 |
| Aspartate aminotransferase increase | 29 | 0 | 34 | 1 |
| Triglyceride increase | 27 | 2 | 15 | 1 |
| Alanine transaminase increase | 17 | 0 | 24 | 1 |
| Sodium decrease | 16 | 1 | 15 | 0 |
| Creatinine increase | 16 | 0 | 6 | 0 |
Discussion
The approval of elacestrant as a subsequent line of therapy for patients with ER+/HER2- metastatic breast cancer offers a novel therapeutic option in a setting that previously had limited options. The results of the EMERALD trial showed that elacestrant offers PFS benefits when compared with monotherapy endocrine therapy in the subsequent-line setting with manageable side effects. Patients can be at risk for dyslipidemia, so fasting lipid panel should be monitored prior to starting therapy and periodically while on therapy.
References
- Breast Cancer. NCCN Clinical Practice Guidelines in Oncology. Version 4.2023.
- American Cancer Society. (2023, March 1) Survival Rates for Breast Cancer. https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html
- 3. Burstein H. J. (2020). Systemic Therapy for Estrogen Receptor-Positive, HER2-Negative Breast Cancer. The New England journal of medicine, 383(26), 2557–2570.
- ORSERDU (elacestrant) [package insert]. New York, NY; Stemline Therapeutics, Inc: 2023.
- U.S. Food & Drug Administration. (2023, August 21) List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools
- Bidard, F. C., Kaklamani, V. G., Neven, P., Streich, G., Montero, A. J., Forget, F., Mouret-Reynier, M. A., Sohn, J. H., Taylor, D., Harnden, K. K., Khong, H., Kocsis, J., Dalenc, F., Dillon, P. M., Babu, S., Waters, S., Deleu, I., García Sáenz, J. A., Bria, E., Cazzaniga, M., … Bardia, A. (2022). Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 40(28), 3246–3256.

Jina Yun, PharmD, is a clinical oncology pharmacist at Fred Hutchinson Cancer Center/University of Washington Medical Center, Seattle, WA.