Alice Wang, PharmD | Dec 8, 2021 11:42:07 AM
On August 13, 2021, the Food and Drug Administration (FDA) expanded the Emergency Use Authorization for the Pfizer and Moderna COVID-19 vaccines to recommend a third dose for certain immunocompromised patients.1 This recommendation was based on data suggesting that these patients experienced reduced antibody responses to the two-dose series, resulting in an increased risk of breakthrough infections and poor outcomes. In fact, immunocompromised individuals make up only 3% of the United States population, yet account for 44% of breakthrough COVID-19 infections.2-3
A recently published analysis of data from the Leukemia and Lymphoma Society National Patient Registry (NCT04794387) reported the vaccination response rates in various hematological malignancies.4
A total of 1,445 patients were included in this analysis. Patients with hematologic malignancies received anti-spike antibody serologic testing at least 14 days after the second dose of an mRNA vaccine during the months of March-May 2021. Patients with prior exposure to COVID-19 were excluded from analysis. The median age of this population was 68 (range, 16-110 years), with the majority being female (60%) and Caucasian (95%). Forty-five percent received the Moderna vaccine, and the median time to serology testing was 42 days.
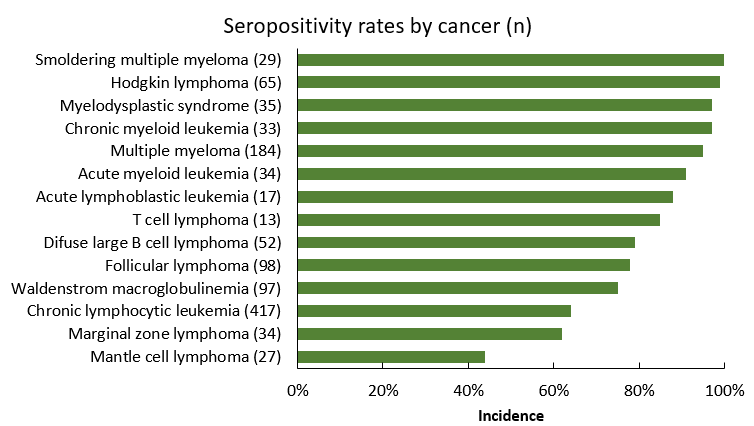
Seventy-five percent of patients were found to have produced antibodies to the mRNA vaccines. This seroconversion rate aligns with previously reported data in hematologic malignancies of 46-85% but stands in contrast to the 100% response in immunocompetent cohorts. Seroconversion was lowest among patients with B-cell malignancies, including mantle cell lymphoma (MCL, 44%), marginal zone lymphoma (MZL, 62%), chronic lymphocytic leukemia (CLL, 74%), and diffuse large B cell lymphoma (DLBCL, 79%).
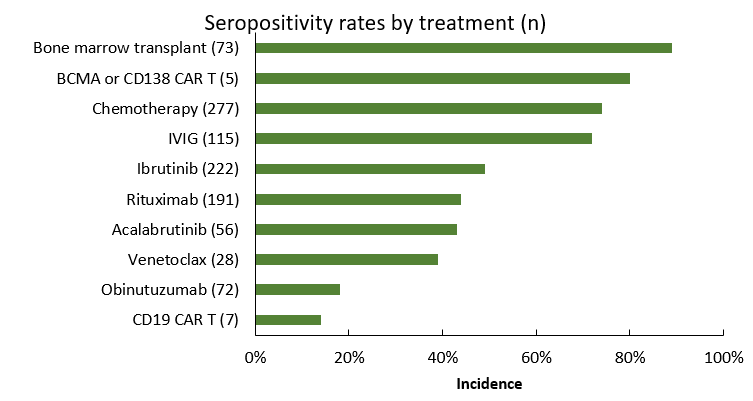
Lower rates of seroconversion were also associated with exposure to B-cell suppressing therapies, such as anti-CD20 antibodies and Bruton tyrosine kinase inhibitors (18-44% and 43-50%, respectively). This is not surprising given the role of B-cell function in cellular immune responses, as well as data for serologic responses to other vaccines in this population. Interestingly, of the seronegative CLL cohort, 28% had not received therapy within 2 years prior, underscoring the impact of disease pathophysiology on antibody response. On the other hand, seroconversion was highest in chronic myeloid leukemia (97%), acute myeloid leukemia (91%), and acute lymphocytic leukemia (88%), as well as multiple myeloma (95%). The incidence of seroconversion after chimeric antigen receptor (CAR)-T therapy was lower in CD19 CAR-T, versus CD138 or BCMA CAR-T therapy (14% vs 80%).
Further analysis of seronegative responses suggests that the Moderna vaccine was associated with higher odds of seroconversion (odds ratio 1.5; 95% confidence interval 1.1-2, p=0.007). This difference remained after regression models adjusting for age, cancer, and gender. Potential contributing factors to this difference include the higher dose of spike mRNA in the Moderna vaccine, and differences in mRNA coding sequences and dosing schedules used.
This analysis was limited by the self-reported nature of data points in the patient registry, including disease and treatment history, as well as the use of seroconversion as a surrogate endpoint for the vaccines’ clinical efficacy. It also did not account for the impact of T-cell mediated responses in immune protection. Nevertheless, this study adds to the body of literature suggesting that patients with hematologic malignancies have poorer responses to the COVID-19 mRNA vaccines and may be at higher risk of breakthrough infections.
As of early November 2021, the CDC continues to recommend a third dose of the COVID-19 vaccine for moderately to severely immunocompromised patients at least 28 days after the second dose of an mRNA vaccine. This recommendation applies to those with active or recent cancer treatment, CAR-T therapy, stem cell transplant within 2 years or taking immunosuppressives, and other immunosuppressive therapy, including high-dose corticosteroid (e.g. >20 mg prednisone for >2 weeks). The purpose of this additional dose is to augment the primary series when the initial immune response may be insufficient. This is in contrast to the booster dose recommended for other high-risk individuals to be given at least 6 months after the primary vaccination series to boost immune response that may have waned over time. Healthcare professionals can find updated information on vaccination recommendations on the CDC website, https://www.cdc.gov/vaccines/covid-19/index.html.5
References:
- 1. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. U.S. Food and Drug Administration. Available at:
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. Accessed November 11, 2021. - 2. Harpaz R, Dahl RM, Dooling KL. Prevalence of Immunosuppression Among US Adults, 2013. JAMA. 2016;316(23):2547-2548. doi:10.1001/jama.2016.16477
- 3. Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. Preprint. medRxiv. 2021;2021.07.08.21259776. Published 2021 Jul 8. doi:10.1101/2021.07.08.21259776
- 4. Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031-1033. doi:10.1016/j.ccell.2021.07.012.
- 5. Interim Clinical Considerations for Use of COVID-19 Vaccines. Cdc.gov. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Published 2021. Accessed November 11, 2021.

Alice Wang, PharmD