Kim Navarrete, MSN, APRN, FNP-C, OCN,
and Nilesh Kalariya, PhD, AGPCNP-BC, AOCNP
Multiple myeloma (MM) is a hematologic malignancy associated with the clonal proliferation of plasma cells. Despite decades of progress with MM therapies, the management of patients with relapsed/refractory MM (RRMM) remains a persistent clinical challenge. B-cell maturation antigen (BCMA) is a cell-surface protein universally and selectively expressed on plasma cells; therefore, is an ideal target for T-cell redirecting therapies for malignant plasma cells.
On October 25, 2022, Tecvayli (teclistamab, Janssen Biotech, Inc.) received FDA approval as the first bispecific antibody for patients with RRMM who have received at least 4 prior lines of therapy and with prior exposure to a proteasome inhibitor, immunomodulatory drug, and anti-CD38 monoclonal antibody. Tecvayli is the third immune therapy directed against BCMA for RRMM with regulatory approval,1 following the recent approval of 2 BCMA-directed chimeric antigen-receptor T-cell (CART) therapies.
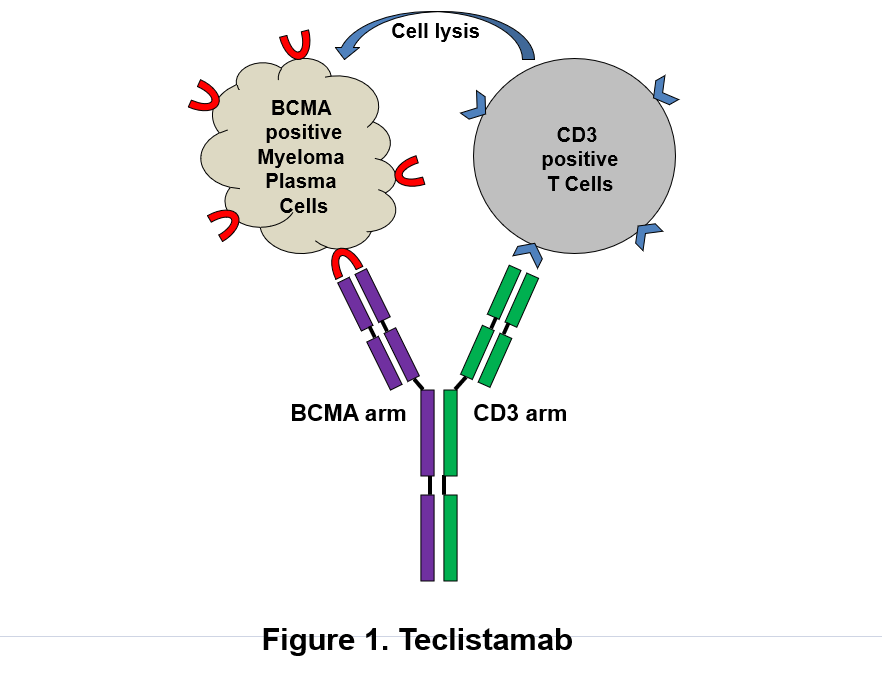
Figure: Schematic depicts dual mechanism of action of Tecvayli (teclistamab, Janssen Biotech, Inc.) against B-cell maturation antigen (BCMA)-expressing plasma cells. (1) Tecvayli directly binds to plasma cells via BCMA and destroys myeloma cells. (2) Tecvayli can also recruit and activate CD3-positive T cells which cause lysis of myeloma cells.
Tecvayli is a humanized IgG4 antibody with dual antigenic specificity. It recognizes the tumor-associated antigen BCMA on plasma cell surface. It can also bind to CD3Ɛ on T lymphocytes leading to T-cell activation and redirecting to BCMA expressing malignant plasma cells subsequently resulting in lysis of tumor cells (Figure). To stabilize and minimize immunological effector functions, its Fc regions contain the S228P/L234A/L235A mutations to reduce affinity to Fcγ receptors.2
MajesTEC-1 Study
Based on results from the pivotal phase 1/2 MajesTEC-1 trial (NCT03145181 and NCT04557098), Tecvayli showed promising efficacy and safety in adult patients with RRMM with manageable safety profile.3 These results from this single-arm, multi-cohort, open-label, multi-center study led to FDA approval of first-in-class, bispecific T-cell antibody for patients with RRMM who received at least 4 prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
The major inclusion/exclusion criteria for the MajesTEC-1 study are listed in the Table. This global study, with 35 sites from 9 countries, enrolled a total of 165 patients who received Tecvayli at the recommend phase 2 dose. The median age was 64 years (range 33 to 84), with 58.2% male. The patients with triple-class and penta-drug refractory disease were 128 (77.6%) and 50 (30.3%), respectively. Among all patients, 28 (17.0%) patients had extramedullary disease, defined as the presence of 1 or more plasmacytoma soft tissue lesions, at baseline. Among the 148 patients with high-risk cytogenetic profile, 38 (25.7%) patients had at least 1 high-risk cytogenetic abnormality including del(17p) (15.5%), t(4:14) (10.8%) and/or t(14;16) (2.7%).3
Table. Select Inclusion/Exclusion Criteria for the MajesTEC-1 Study
| Inclusion Criteria | Exclusion Criteria |
| – ≥18 years of age – Documented of initial diagnosis of MM according to IMWG diagnostic criteria – Documented relapsed or refractory MM with ≥3 lines of MM treatment, including triple-class exposure to an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody – Hemoglobin ≥8 g/dL, platelets ≥75×109/L – Absolute neutrophil count (ANC) ≥1.0×109/L – Serum creatinine clearance ≥40mL/min/1.73 m2 | – Prior anti-BCMA therapy or any other CD3-redirecting drug – Plasma cell leukemia, Waldenstrom’s macroglobulinemia, POEMS syndrome, or amyloidosis – History of stroke, seizure, allogeneic stem cell transplantation within the past 6 months -Known active CNS involvement or exhibits clinical signs of meningeal involvement MM – Active or documented history of autoimmune disease, except for vitiligo, Type 1 diabetes, and prior autoimmune thyroiditis – Eastern Cooperative Oncology Group (ECOG) Performance Status ≥2 |
Source: Tecvayli Prescribing Information.
With a median follow up of 14.1 months, the overall response rate was 63%, which included 39.4% of patients with complete remission (CR) or better, 19.4% of patients with very good partial response and 4.2 % of patients with partial response. The minimal residual disease rate was 46% among patients with a CR or better. The median duration of response and median duration of progression-free survival were 18.4 months (95% confidence interval (CI), 14.9 to not estimable) and 11.3 months (95% CI, 8.8 to 17.1), respectively.3
Adverse Events and Boxed Warning
All study participants exhibited an adverse event (AE). However, 94.5% patients had grade 3 or grade 4 adverse events. The hematologic toxicities were the most common in these patient population with 70.9% with neutropenia, 52.1% with anemia, and 40% with thrombocytopenia. The patients who reported infections and hypogammaglobulinemia were 76.4% and 74.5%, respectively. The cytokine release syndrome (CRS) occurred in 72.1% of patients. The CRS mostly occurred in cycle 1 dosing period. Most CRS events were grade 1 or 2 and subsequently resolving completely. Neurotoxic events, including immune effector cell-associated neurotoxicity syndrome occurred in 14.5% patients; most events were grade 1 or 2 with one grade 4.3
Based on toxicity data, the boxed warning for Tecvayli prescribing information included CRS and neurologic toxicity, including immune effector cell-associated neurotoxicity (ICANS).4 Therefore, Tecvayli is available only as part of a risk evaluation and mitigation strategy (REMS), called the Tecvayli REMS that requires specific training and AE reporting.4
Tecvayli is available in liquid solution in vials containing 30 mg in 3 mL (10 mg/mL) and 153 mg in 1.7 mL (90 mg/mL). The recommended regimen is a step-up subcutaneous injection schedule of 0.06 mg/kg on Day 1, 0.3 mg/kg on Day 4, and 1.5 mg/kg on Day 7, followed by 1.5 mg/kg once weekly until disease progression or intolerance.4 Additionally, Tecvayli has been included in the National Comprehensive Cancer Network (NCCN) Guidelines for treatment of RRMM.5
Compared to CAR T-cell therapy which requires 4 to 6 weeks for T-cell collection followed by CAR T-cell manufacturing, the advantage of Tecvayli is that it allows for immediate dosing in an “off-the-shelf” format. This advantage would hopefully provide more patients access to BCMA-targeted bispecific therapies.


References
- Hua, G., Scanlan, R., Straining, R., Carlson, D., (2023). Teclistamab-cqyv: The First Bispecific T-Cell Engager Antibody for the Treatment of Patients With Relapsed or Refractory Multiple Myeloma. Journal of the Advanced Practitioner in Oncology, 14(2): 163-171.
- Pillarisetti, K., Powers, G., Luistro, L., Babich, A., Baldwin, R., Li, Y., . . .Gaudet, F. (2020). Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Advanced; 4(18): 4538-49.
- Moreau, P., Garfall, A.L., Donk, N.W., Nahi, H., San-Miguel, J.F., Oriol, A., . . . Usmani, S.Z. (2022). Teclistamab in Relapsed or Refractory Multiple Myeloma. The New England journal of medicine, 387(6),495-505.
- Janssen Biotech Inc. (2022). TECVAYLI Prescribing information. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761291s000lbl.pdf
- National Comprehensive Cancer Network. (2022). Multiple Myeloma (version 3.2023). Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf