Nilesh Kalariya, PhD, AGPCNP-BC, AOCNP
Multiple myeloma (MM) is the second most common hematological malignancy; it is characterized by the clonal proliferation of plasma cells, and it is incurable. Despite therapeutic advances in the past 2 decades, the management of relapsed and/or refractory MM continues to be a huge challenge. B-cell maturation antigen (BCMA) is a cell-surface receptor expressed on normal and malignant plasma cells, thereby making it a suitable therapeutic target in MM. On March 26, 2021, Abecma (idecabtagene vicleucel, Bristol Myers Squibb) received FDA approval becoming the first anti-BCMA chimeric antigen receptor T-cell (CAR T) therapy for relapsed or refractory MM patients.1
Abecma is a genetically modified autologous T-cell product transduced with lentiviral vector expressing a CAR-targeting BCMA. The CAR construct comprises a tumor binding domain (a murine extracellular single-chain variable fragment specific for BCMA), a linker (a human CD8α hinge), and a T-cell intracellular signaling chain (transmembrane domain with 4-1BB and CD3ζ). The CAR construct binds to BCMA-expressing target cells via a tumor binding domain leading to signaling through the signaling chain and subsequent activation of CAR T cells. The activation of CAR T cells triggers T cell proliferation, cytokine secretion, and, subsequently, cytolytic killing of target cells.
KarMMa Study
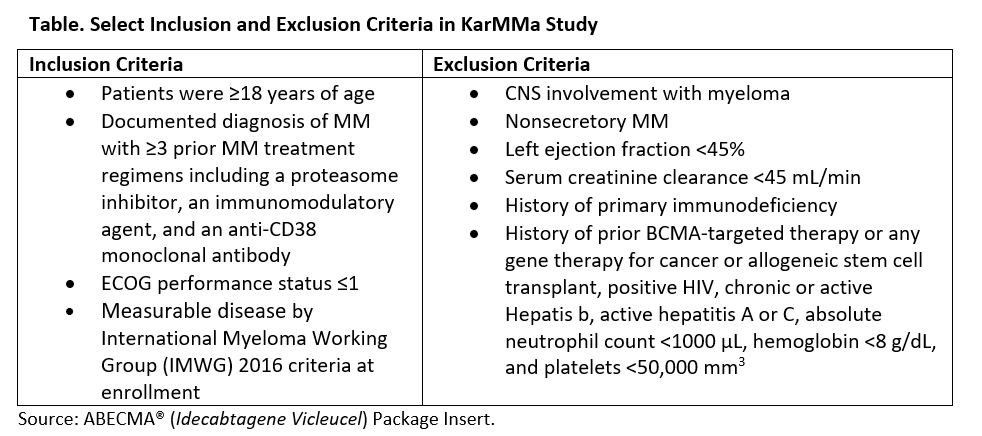
The FDA approval of Abecma was based on the pivotal KarMMa study, which is an open label, single arm, multicenter phase 2 clinical trial (NCT03361748).There were very specific inclusion/exclusion criteria for eligibility which are listed in the Table
The study enrolled a total of 140 patients; 128 patients received CAR T-cell infusion and 12 patients left the study before CAR T-cell infusion.2 Among 128 evaluable patients, the average age was 61 years (range 33-78), and 59% were male. Almost 98% of the patients had an Eastern Cooperative Oncology Group (ECOG) performance status score ≤1. The median number of prior anti-MM therapies was 6 (range 3-16). The patients’ refractory disease status included double- (89%), triple- (84%), and penta-refractory disease (26%). Based on the revised MM International Staging System (R-ISS), the percentage of the patients with stage I, or stage II, or stage III was 11, 70, and 16 respectively. The percentage of patients with high-risk cytogenetics abnormality were 35%, which included del17p (18%), t(4;14) (18%), and t(14;16) (5%). Some of the patients had more than 1 cytogenetics abnormality. The other notable cytogenetics abnormalities were 1q amp, 13q34 monosomy, 13q14 del, and 1p del.
The overall response rate (ORR) was 73% (range 66%-81%) (95% confidence interval [CI]) among which 33% had either a complete response or a stringent complete response, and total of 52% had very good partial response or better. Of the 33% patients with a complete or stringent complete response, 79% had negative minimal residual disease (MRD).
Adverse Events
The pivotal KarMMa study reported that 99% of the patients had grade 3 or higher adverse events.2 Most of these higher-grade adverse events were hematological, including 89% neutropenia, 60% anemia, 52% thrombocytopenia, 39% leukopenia, 27% lymphopenia, and 16% febrile neutropenia. Any grade cytokine release syndrome (CRS) and neurotoxic effects were observed in 84% and 18% of the patients, but only 5% and 3% of the patients had grade 3 or higher, respectively. Hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS) was observed in 4% of the patients. Most adverse events occurred within 8 weeks post CAR T-cell infusion. Based on toxicity data, the boxed warning for Abecma prescribing information included CRS, neurotoxic effects, HLH/MAS, and prolonged cytopenias.3 The other warnings also include serious infections, hypogammaglobulinemia, secondary malignancies, and hypersensitivity reactions.
The FDA approved Abecma for adult patients with relapsed or refractory multiple myeloma who have received 4 or more prior lines of therapy including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody. The eligible relapsed/refractory patient population on the pivotal KarMMa study received at least 3 prior lines of therapies. However, FDA restricted approval to patients with 4 prior lines based on 88% efficacy whereas the benefit vs risk data were limited for patients with 3 prior lines. The recommended dose for Abecma is 300 to 460 x 106 CAR T cells. Abecma is now provided as a single intravenous infusion in 1 or more bags. CAR T therapy has also been incorporated in the National Comprehensive Cancer Network (NCCN) Guidelines for MM as an option for patients with relapse or progressive disease.4
Nilesh Kalariya, PhD, AGPCNP-BC, AOCNP, is a nurse practitioner in the Department of Lymphoma/Myeloma at The University of Texas MD Anderson Cancer Center.

Nurse Practitioner,
The University of Texas MD Anderson Cancer Center,
Department of Lymphoma/Myeloma
1515 Holcombe Blvd, Unit 0429,
Houston, TX 77030-4009
Email: NMKalariya@mdanderson.org
References
- Sharma, P., Kanapuru, B., George, B., Lin, X., Xu, Z., Bryan, W. W., Pazdur, R., & Theoret, M.R. (2022). FDA Approval Summary: Idecabtagene Vicleucel for Relapsed or Refractory Multiple Myeloma. Clin Cancer Res, 28(9), 1759-1764.
- Munshi, N.C., Anderson, L.D. Jr., Shah, N., Madduri, D., Berdeja, J., Lonial, S., …San-Miguel, J. (2021). Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. The New England journal of medicine, 384(8), 705-716.
- 2021. ABECMA® (Idecabtagene Vicleucel) Package Insert. Available at https://www.fda.gov/media/147055/download
- NCCN Guidelines® for Multiple Myeloma. Version 1.2023. Available at https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf