Nilesh Kalariya, PhD, AGPCNP-BC, AOCNP, and Amy Barker, MSN, APRN, FNP-C
We have witnessed dismal overall outcomes in individuals with relapsed and/or refractory (R/R) multiple myeloma (MM) for many years. However, the scientific advancement in the past few years led to the FDA approval of 5 new agents belonging to 2 new categories called chimeric antigen receptor (CAR) T-cell and bispecific T-cell engager (BiTE) therapies. These therapies have shown deep and durable responses in patients heavily pretreated for multiple myeloma. Identifying new targets and therapeutic agents in MM provides excitement and hope that a sustained response and eventually a cure could be achievable. In this article, we will review the efficacy and safety of the FDA-approved CAR-T and BiTE therapies.
CAR T-Cell Therapies
Two B-cell maturation antigen (BCMA)–directed autologous CAR T-cell therapies, Abecma [Idecabtagene vicleucel, Bristol Myers Squibb] and Carvykti [ciltacabtagene autoleucel, Janssen Oncology] have been approved for heavily pretreated patients with R/R MM with at least 4 prior lines of therapy.1,2 The pivotal study data on safety and efficacy have been impressive and reported in the literature with overall response rate (ORR) of 66% to 99%. Moreover, the real-world data on safety and efficacy from the patients who received CAR T-cell therapies under a commercial label was recently published and it shows a comparable safety and efficacy profile.
It is interesting to note a few crucial findings of the pivotal studies as well as follow up for Abecma and Carvykti. Abecma reported 26% patients achieving minimal residual disease (MRD) negativity within 3 months post CAR-T cell infusion1 and progression-free survival (PFS) of 13.3 months.3 In the study population, 35% of patients had high-risk cytogenetics and 39% had extramedullary disease (EMD). The studies that included Carvykti reported 57% patients with 27 months PFS.4 The study population had 24% patients with high-risk cytogenetics and 13% patients with extramedullary plasmacytoma. Thus, CAR T-cell therapy holds promise for hard-to-treat patients and offers novel dual advantages: a single infusion and off-treatment period. The efficacy and safety data for BCMA-directed CAR T-cell therapies have led to this becoming standard of care choice of physicians for the treatment of R/R MM.
Bispecific T-cell Engager Antibodies
Over the past 13 months there have been 3 BiTE antibodies approved by FDA for use in R/R MM which include Tecvayli [teclistamab-cqyv, Janssen Oncology], Talvey (talquetamab-tgvs, Janssen Oncology], and Elrexfio [elranatamab-bcmm, Pfizer]. Tecvayli and Elrexfio target BCMA on plasma cells and CD3 on T cells while Talvey targets G-protein coupled receptor class C group 5 member D (GPRC5D) on plasma cells and CD3 on T cells. Like CAR T-cell therapy, the ORR reported by BiTEs’ pivotal studies, 50% to 70%, were also robust.5,6,7
A considerable number of patients achieved complete remission (CR) or better and MRD negativity. It is interesting to note that a response was also observed in patients with high-risk cytogenetics, EMD, triple-class-refractory, penta-refractory, and previous BCMA-targeted antibodies or CAR T-cell therapies. Bispecific antibodies are readily available with convenient subcutaneous (SQ) administration and approved for patients with R/R MM who received 4 prior lines of therapy.
Safety
Some of the most common side effects of CAR T-cell and BiTE therapies include cytokine release syndrome (CRS), neurotoxicity, hematologic toxicity (cytopenias), infections, hepatotoxicity, and hypogammaglobulinemia. The onset and resolution of CRS have been noted within the first week or 2 after these therapies and occurred in majority of the patients but were generally of grades 1 and 2.
The incidence of neurotoxicity has been low compared to CRS and hematologic toxicity with these therapies. However, late onset neurotoxicity could occur including parkinsonian-like symptoms and cranial neuropathies especially after Carvykti. Therefore, close monitoring is warranted for early detection and an intervention for such patients. The hematologic toxicity, generally reversible, includes anemia, thrombocytopenia, and neutropenia. It is important to note that cytopenias may continue for 2 to 3 months post CAR T-cell therapies requiring close monitoring. Higher risk and incidence of infections have been observed. Because these therapies target normal as well as malignant plasma cells, hypogammaglobulinemia has been reported to occur in a considerable number of patients.
Besides these common side effects, a few sporadic cases of dose-limiting toxicities with BiTE have been reported in the literature. Therefore, a watchful approach is warranted to recognize such toxicities early on followed by a decision to discontinue treatment permanently or to resume after resolution of toxicity.
Due to continuous and multiple subcutaneous injections required for BiTE therapy administration, one should also be monitoring for injection site reactions. Because of the presence of GPRC5D in off-target tissues, target-specific side effects with Talvey have been observed in skin, nails, and oral cavity. Nevertheless, the toxicities associated with CAR T-cell or BiTE therapies have been manageable and largely reversible. Full physical examination at baseline prior to starting treatment and at regular intervals, as well as reinforcing patient education at each encounter to watch for potential signs and symptoms, are some of the clinical strategies that should be employed by the providers to identify toxicities at the earliest.
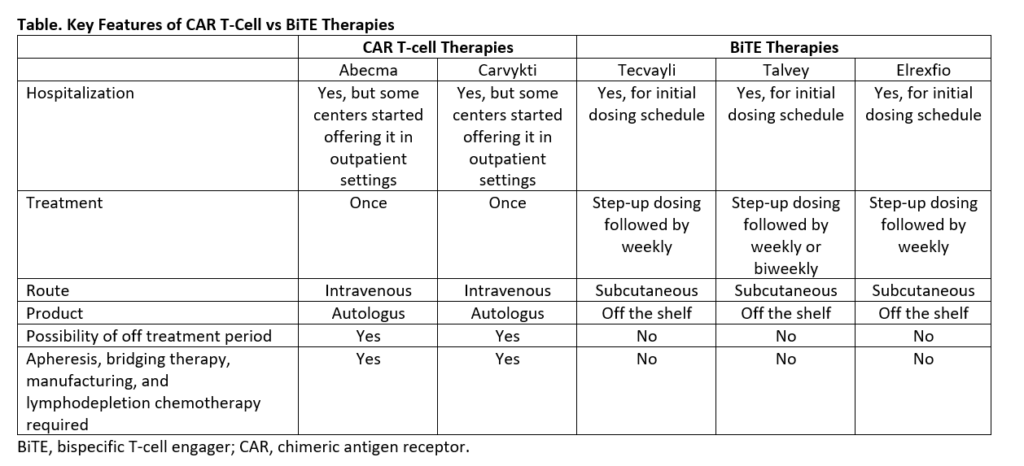
With availability of multiple novel agents, selection of an appropriate agent is a new challenge that we encounter now. A comparative look at key features of these therapies highlighted in the Table helps patients and providers to navigate through treatment decision making. In addition, clinical, and investigational efforts are in progress which may provide robust scientific data prompting change in FDA approval to allow use of these therapies in earlier or frontline settings.

Nilesh Kalariya, PhD, AGPCNP-BC, AOCNP

Amy Barker, MSN, APRN, FNP-C
References:
- Munshi, N. C., Anderson, L. D., Jr, Shah, N., Madduri, D., Berdeja, J., Lonial, S., Raje, N., Lin, Y., Siegel, D., Oriol, A., Moreau, P., Yakoub-Agha, I., Delforge, M., Cavo, M., Einsele, H., Goldschmidt, H., Weisel, K., Rambaldi, A., Reece, D., Petrocca, F., … San-Miguel, J. (2021). Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. The New England journal of medicine, 384(8), 705–716.
- Berdeja, J. G., Madduri, D., Usmani, S. Z., Jakubowiak, A., Agha, M., Cohen, A. D., Stewart, A. K., Hari, P., Htut, M., Lesokhin, A., Deol, A., Munshi, N. C., O’Donnell, E., Avigan, D., Singh, I., Zudaire, E., Yeh, T. M., Allred, A. J., Olyslager, Y., Banerjee, A., … Jagannath, S. (2021). Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet (London, England), 398(10297), 314–324.
- Rodriguez-Otero, P., Ailawadhi, S., Arnulf, B., Patel, K., Cavo, M., Nooka, A. K., Manier, S., Callander, N., Costa, L. J., Vij, R., Bahlis, N. J., Moreau, P., Solomon, S. R., Delforge, M., Berdeja, J., Truppel-Hartmann, A., Yang, Z., Favre-Kontula, L., Wu, F., Piasecki, J., … Giralt, S. (2023). Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. The New England journal of medicine, 388(11), 1002–1014.
- Martin, T., Usmani, S. Z., Berdeja, J. G., Agha, M., Cohen, A. D., Hari, P., Avigan, D., Deol, A., Htut, M., Lesokhin, A., Munshi, N. C., O’Donnell, E., Stewart, A. K., Schecter, J. M., Goldberg, J. D., Jackson, C. C., Yeh, T. M., Banerjee, A., Allred, A., Zudaire, E., … Jagannath, S. (2023). Ciltacabtagene Autoleucel, an Anti-B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 41(6), 1265–1274.
- Chari, A., Minnema, M. C., Berdeja, J. G., Oriol, A., van de Donk, N. W. C. J., Rodríguez-Otero, P., Askari, E., Mateos, M. V., Costa, L. J., Caers, J., Verona, R., Girgis, S., Yang, S., Goldsmith, R. B., Yao, X., Pillarisetti, K., Hilder, B. W., Russell, J., Goldberg, J. D., & Krishnan, A. (2022). Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. The New England journal of medicine, 387(24), 2232–2244.
- Moreau, P., Garfall, A. L., van de Donk, N. W. C. J., Nahi, H., San-Miguel, J. F., Oriol, A., Nooka, A. K., Martin, T., Rosinol, L., Chari, A., Karlin, L., Benboubker, L., Mateos, M. V., Bahlis, N., Popat, R., Besemer, B., Martínez-López, J., Sidana, S., Delforge, M., Pei, L., … Usmani, S. Z. (2022). Teclistamab in Relapsed or Refractory Multiple Myeloma. The New England journal of medicine, 387(6), 495–505.
- Lesokhin, A. M., Tomasson, M. H., Arnulf, B., Bahlis, N. J., Miles Prince, H., Niesvizky, R., Rodrίguez-Otero, P., Martinez-Lopez, J., Koehne, G., Touzeau, C., Jethava, Y., Quach, H., Depaus, J., Yokoyama, H., Gabayan, A. E., Stevens, D. A., Nooka, A. K., Manier, S., Raje, N., Iida, S., … Mohty, M. (2023). Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nature medicine, 29(9), 2259–2267.